Technical Manual
For the VIS INFLUERE® Medical Device
Content
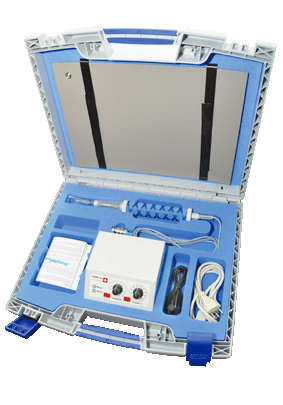
1.1 Picture of the device in the case and the individual parts
1.2 Scope of the delivery and commissioning
1.3 Application
1.4 Ending the application
1.5 Care and maintenance
1.6 Warnings
1.7 Guarantee and repair conditions
1.8 Electromagnetic compatibility
1.9 Disclaimer
1.10 Production batch
1.11 About us/Imprint
1.12 Technical data
VIS INFLUERE® Medical is a HI-TECH device with encrypted software. Made in Switzerland
Safety marks, warnings
![]() Device of Protection Class II
Device of Protection Class II
![]() Operation instructions
Operation instructions
![]() application part Type BF
application part Type BF
IP 00 not protected agains penetration of foreign bodies or against the ingress of water
1.2 Scope of the delivery and commissioning
Contents of the Vis-Influere® case:
• Vis-Influere® rod with protective cap and connecting cable
• Control unit (mains power adapter) with the electronic controller and LED display,
• Mains cable
• Contact mat with cable for connection to the control unit
• Oil (intended for use in case of any reddening after the treatment)
• Instruction manual
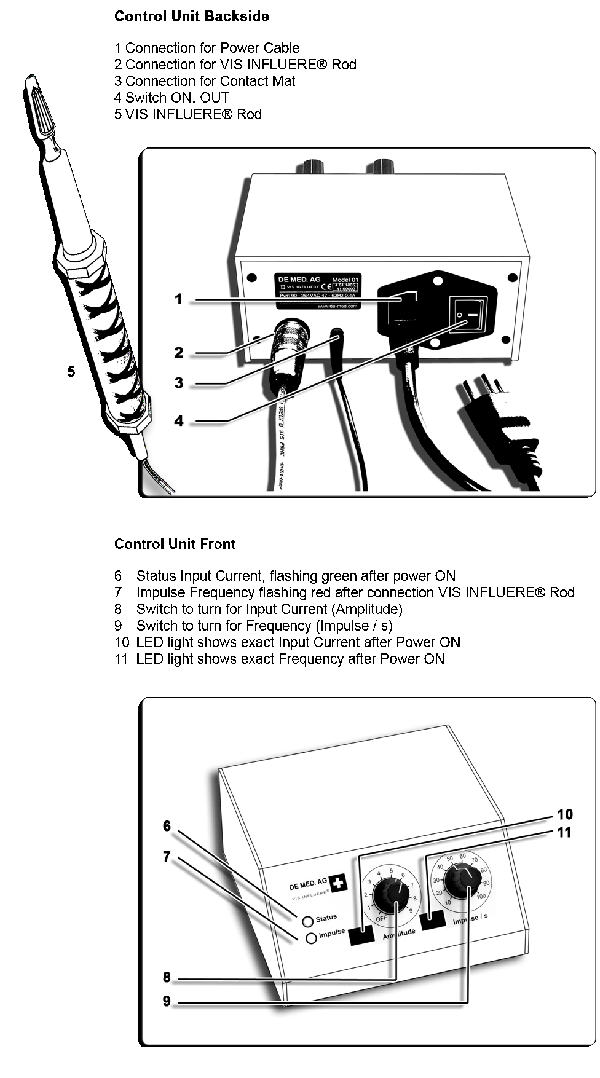 Commissioning:
Commissioning:
Plug the control unit power cable into an earthed mains socket with the switch set to OFF. Connect the contact mat cable to the control unit und contact mat. Connect the Vis-Influere® rod to the control unit, screw in the connection and remove the protective cap, Caution: Place the cables, so that the mains cable can be easily pulled and that tripping over the cables is avoided Set the switch to ON. Basic settings: Set the frequency to 50 hertz and set the amplitude to approx. 4.0.
Please observe the instructions for the settings
If the amplitude is set to a value below 1, the Vis-Influere® rod will be switched off.
This provices the user with the possibility of switching off the instrument during an interruption, without switching off the control unit.
The values of the LED display that can be seen on the setting scale for frequency and amplitude are always correct.
Due to the principle of operation, a crackling sound with varying intensity will be heard during operation, and bluish lights will be seen in the glass tip.
1.3. Application
VIS INFLUERE® (VI) is a high frequency device of the latest design.
VI generates high-frequency currents, i.e. electrical alternating currents with very high frequencies (around 1 to 2 million per second). The whole device unit consists of a control unit with LED display for amplitude and impulse, the VI rod containing a hand-blown glass tip with a quartz crystal and argon gas. This glass tip is also referred to as an electrode which generates ultraviolet radiation around the specially ground pure crystal. As opposed to other high frequency devices, the VI has been developed as an ‘all-in-one’ device. VI is characterised specially by its mobility.
The vasodilator effect of the high frequency (H) stimulates the whole human organism and optimises the function of the organs; i.e. there is a better blood circulation and an increase in the oxygen content of the blood and tissue. The VI-H creates an acceleration of the metabolism, has a bactericidal and germicidal effect and releases ozone as a result of weak, harmless spark discharges. The application areas of the VI-H are practically unlimited, apart from the fact that the VI-H can only be used for non-invasive applications.
The overall purpose of VI:
a. widening of vessels
b. stimulation of the organism
c. increased and improved blood flow
d. increase of oxygen content in blood and tissue
e. encourage of metabolism
General information:
The Vis-Influere® rod is applied directly or indirectly to the skin and hair on the body. It must not be used in the eyes or in body orifices. Any use in the general area of the eyes (close use only with protective goggles!) and on the mucous membrane should be strictly avoided.
In addition, the device must not be used in the following cases:
• When using a pacemaker,
• Persons who carry medicinal devices with metal components in or on their body, such
as insulin pumps or neurostimulators, or patients with heat valves, vascular clips, stents or shunts
• Persons with heart problems or nerve damage of any kind
• In the case of pregnant patients, no treatment of the abdominal area or points that could
potentially induce contractions.
The device should only be switched on during the treatment. It should otherwise always be in the OFF mode. The device must be kept out of the reach of children at all times.
1.4. Ending the application
Switch the device off, and secure the Vis-Influere® rod with the protective cap. Store the Vis-Influere® rod safely, preferably in the supplied case, so that the glass on the tip will not be damaged.
Disconnecting the cables from the control unit: disconnect power cable, disconnect grounding mat cable, and disconnect the Vis-Influere® rod cable. The glass tip must be kept secure so that no damage is possible
1.5. Care and maintenance
For reasons of hygiene, the tip of the Vis-Influere® rod must be cleaned after every use with alcohol swabs or a soft cloth and a conventional disinfectant. The housing of the control unit and the Vis-Influere® rod can be easily cleaned using a damp cloth.
The housing material is resistant to conventional disinfectants used for surface disinfection. Under no circumstances should you use detergents containing solvents, acetone, petrol or similar agents to clean the device. Solvents will damage the surface of the control unit or the Vis-Influere® rod, and could render the device permanently unusable.
Do not immerse the Vis-Influere® rod in water.
1.6. Warnings
- Protect the device from moisture.
- Do not operate the device without the enclosed contact mat.
- When not in use, switch the device off and disconnect it from the mains supply.
- The device may only be operated with a correctly grounded power socket.
- Never operate the device with a defective Vis-Influere® rod, as there is the danger of electric shock, and do not operate the Vis-Influere® rod with a defective connecting cable.
- The device may only be opened by a specialist from DE MED. AG.
- There are no components in the device that can be serviced or replaced by the user.
- Exception: Changing the glass tip if this is damaged, but only in the OFF mode. Remove the tip by
- unscrewing (as for a light bulb) and replace it with a replacement tip from De MED AG.
- Never cover the housing of the control unit when it is in operation – danger of overheating!
- The housing of the control unit may become warm to the touch during a longer period of operation. This is not dangerous, however.
- Do not bring the glass tip close to other electrical or electronic devices, as they could be damaged.
- The device must always be kept out of the reach of children
- Modifying the device id NOT permitted
1.7. Guarantee and repair conditions
DE MED. AG provides a 12-month guarantee from the date of purchase. This covers material and manufacturing defects. Consumables, normal wear and tear, and damage to any parts as a result of incorrect use or external influences are excluded from the guarantee. Special care must be taken to protect the glass tip of the Vis-Influere® rod. The quartz crystal on the tip is heavier than the glass. If its broken, you have to buy a new one.
The Vis-Influere® rod may only be operated with parts that have been explicitly approved for this by the DE MED. AG company, otherwise the guarantee and the warranty will become null and void. In case of repair work or claims under the guarantee, please contact the Support department of DE MED. AG.: This email address is being protected from spambots. You need JavaScript enabled to view it.
Disposal Information
The device has an electronic module, and can be disposed of at a collection point for electronic devices in the same way as, for example, computers or consumer electronics.
1.8. Electromagnetic compatibility
The complete device is electromagnetically harmless.
1.9. Disclaimer
This device has been developed exclusively for professional use in practices, clinics, institutes, out-patients’ departments and wellness and training centres as a valuable complement to known pain treatment methods. It may only be used by instructed specialists. The use of the device for private purposes takes place at one’s own risk.
The user should explain the process (and any contra-indications) to every patient before the first application, e.g. ozone formation and the harmless crackling sound at the tip of the glass when touching the skin.(and should document both the state of health of the patient and the progress of the course of treatment).
The application takes place at one’s own responsibility. We would emphasize in particular that the time between two applications to the same part of the patient’s body must not be less than 20 minutes , and that the device must always be secured against unauthorised operation.
It is possible that e.g. slight reddening of the skin occurs that afterwards rapidly disappears. These places can simply be rubbed with a little massage oil.
(The treatment with Vis-Influere® is not intended as a replacement for any medical treatment / consultation and, in the case of infirmities, should not lead to the interruption of treatment by a doctor or a health practitioner). A physician should be immediately consulted if any complaints appear during or after the treatment.
The DE MED. AG company accepts no liability for any undesired consequences that arise from the incorrect use of the device or from deviations from the indicated method.
1.10 Product Batch
Each individual device has a unique number from which production batch it originated and was produced.
1.11 Imprint
DE MED. AG
Langmauerstrasse 70, 8006 Zürich, Switzerland
Telefon: 0041 44 586 65 30, www.de-med.com
Suport / Customer service: This email address is being protected from spambots. You need JavaScript enabled to view it.
1.12 Technical data
| Input voltage Range | 100 - 240 VAC |
| Input Frequency Range | 50 - 60 Hz |
| Input Current | 0.4 A max at 90 VAC |
| Operating Power Consumption | 12 W |
| Voltage 250 V / Current: 1.0A / Charakteristics: Flink F | breaking capacity 35 A |
| Working Temperature Range | 0°C – 40°C |
| Storage Temperature Range | -20°C – 60 °C |
| Air pressing by work | 70 kPa -- 106 kPa |
| Storage | 50 kPa -- 120 kPa |
| Cooling | Natural Convection |
| Operating Humidity | 10 – 90 % |
| Relative Humidity | non-condensing |
| Protection against dust and water | IP 00 |
| Medical Certification-Number | CE 0633 |
| Declaration of Conformity | |
| Certification, Berlin Cert, Vis Influere rod |